|
Being dengue-wise!:
Long wait for Dengvaxia
by Rukshana Rizwie
While Sri Lanka is one of the hardest hit countries due to by the
rising number of dengue cases, Sri Lankans could may have a long wait
before they get easy and affordable access to a new vaccine that has
just being introduced to the market.
  The world's first dengue vaccine - 'Dengvaxia' - was approved after
20 years of research earlier this month. The vaccine which won clearance
in Mexico is considered a breakthrough in preventing the mosquito-borne
infection that puts half of the world's population at risk. The world's first dengue vaccine - 'Dengvaxia' - was approved after
20 years of research earlier this month. The vaccine which won clearance
in Mexico is considered a breakthrough in preventing the mosquito-borne
infection that puts half of the world's population at risk.
Dengvaxia which was developed at a cost of US$ 1.65 billion was
endorsed by the Mexican regulatory authority for patients between 9 - 45
years, in areas where the disease is endemic. However, the vaccine
awaits approval in at least 19 countries before it can be administered
to patients. This list also includes Sri Lanka.
"It will be a long time before we can administer the vaccine here,"
said Dr. Paba Palihawadana, Chief Epidemiologist and Director of the
Central Epidemiological Unit at the Ministry of Health. "We will
certainly not have it this month. It would most probably be towards the
latter part of next year."
Local testing
Dr. Palihawadana explained that the Ministry of Health would be
required to do a clinical trial in Sri Lanka before it can be made
available locally. She emphasized that details regarding this trial were
not finalized and further discussions were on. "Ordinarily when there is
a new vaccine, part of the Phase III is trials in various countries.
According to the manufacturer (Sanofi Pasteur), it has been cleared in
Asia and Latin America, but we will need to do a litmus test here
locally."
Dengvaxia 'will be priced at a fair, affordable, equitable and
sustainable price, some countries may distribute it free," Guillaume
Leroy, Vice-President of the Dengue vaccine team at Sanofi was quoted as
saying.
"The vaccine has to be registered globally while the World Health
Organization will need to pre-qualify it for use," Dr. Palihawadana
explained, adding that the paper work will cause a snag in releasing the
vaccine on a global scale.
 Meanwhile, Aparna Thomas, a Senior Director of Communications for
India and South Asia in an exclusive email interview to the Sunday
Observer disclosed that the "Company intends to pursue licensure in more
countries in Asia, including Sri Lanka, and will continue to communicate
in a transparent manner regarding our regulatory approval plan for the
vaccine." Meanwhile, Aparna Thomas, a Senior Director of Communications for
India and South Asia in an exclusive email interview to the Sunday
Observer disclosed that the "Company intends to pursue licensure in more
countries in Asia, including Sri Lanka, and will continue to communicate
in a transparent manner regarding our regulatory approval plan for the
vaccine."
She added that "Asia is an impacted region representing approximately
70% of the global burden of dengue, hence Sanofi Pasteur is committed to
delivering this vaccine, upon approval, to Asian endemic countries as
well...."
She said the company would work closely with relevant health
regulatory authorities to secure the approval and access to the vaccine.
"The regulatory file for Dengvaxia(r) has been submitted in 12 endemic
countries - in Latin America and Asia. By the end of the year, we will
have filed for registration in about 20 countries."
WHO discussions
The WHO Strategic Advisory Group of Experts (SAGE) on Immunization is
currently reviewing the evidence for CYD-TDV (Dengvaxia) and will advise
the WHO on a policy position for the vaccine.
Their considerations include; vaccine safety, efficacy, disease
burden, programmatic suitability and cost-effectiveness.
It is expected that this team will discuss the vaccine at its April
2016 meeting and only then provide recommendations to the
Director-General, WHO on the public health utility and any
recommendations for use.
In addition to Dengvaxia, there are five other vaccine candidates
under evaluation in clinical trials, including live-attenuated vaccines,
subunit, DNA and purified inactivated vaccine profiles. Additional
technological approaches, such as virus-vectored and VLP-based vaccines,
are under evaluation in preclinical studies.
However, the WHO in its website notes that the growing global
epidemic of dengue is of mounting concern for the organization while a
safe and effective vaccine is urgently needed. The vaccine will be
integrated as part of the Global Dengue Prevention and Control Strategy
(2012-2020).
Meanwhile, Sanofi Pasteur in a statement published in the Indian
press cited that the vaccine would be offered in India only after the
Sanofi Pasteur receives regulatory approval. So far, the vaccine has
only been granted approval to sell in Mexico.
| [Dengvaxia ]
|
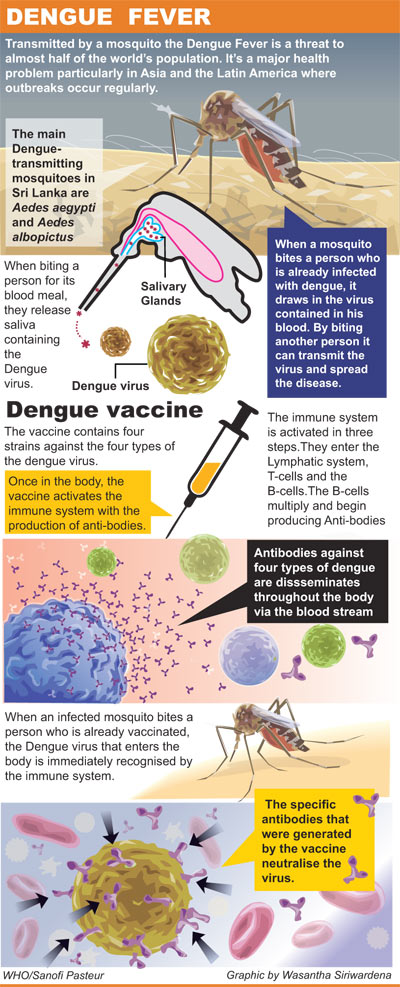
| Dengvaxia is a tetravalent vaccine, which offers protection against
all four virus serotypes. While the dengue virus circulates in only
these four serotypes (types 1-4), the prevalence of each type varies
from outbreak to outbreak.
The Dengue virus exists as four serotypes (Dengue 1-4) and is
genetically related to other flavi viruses such as yellow fever and
tick-borne encephalitis viruses. The most severe form of the disease is
dengue haemorrhagic fever, which is characterized by bleeding and shock.
Aedes aegypti mosquitoes, considered most active during the day, are
usually found near human dwellings and are often indoors. Elevated
temperatures significantly shorten the incubation periods for the dengue
virus in mosquitoes. |
The vaccine, which is being manufactured in France, is ready to be
shipped to India, the company stated, but it was up to Indian regulators
to decide when that would happen.
In this backdrop, a study on the efficacy of the vaccine which was
published in the New England Journal of Medicine in March this year
mentioned three phase III trials on 35,000 children between 2 and 16
years of age, in Asia Pacific and Latin American countries.
The study found that the risk of hospitalisation with dengue fell in
patients aged nine and above, compared to the control group. Based on
the advice from the technical group on the trials, the WHO noted in 2014
that the vaccine's efficacy against all dengue serotypes combined was
estimated at 60.8%.
Trials also showed the vaccine is more effective in people who have
been diagnosed with dengue previously and not so much in unexposed
populations.
Meanwhile, in Sri Lanka, during the past 11 months of this year, some
26,662 suspected dengue cases have been reported to the Epidemiology
Unit from all over the island.
Dr. A. R. M. Thowfeek, Director of the National Dengue Control Unit (NDCU),
said that there had been a 'detectable trend' in the number of cases
reported from the Colombo district after the rains.
"We've noticed a spike in cases at several Municipal area including
Dehiwala - Mt. Lavinia, Battaramulla, Kotte and Kolonnawa," he said.
"We've intensified the anti-dengue measures in those Municipalities."
He added that the NDCU has intensified precautionary measures to
check the incidence of dengue in those towns. He added that one of the
biggest factors was complacency on the part of the residents.
Dr. Thowfeek added that the Director General of Health Services, Dr.
Palitha Mahipala, has called for meetings this week for assistance from
the Sri Lanka Army, the Ministry of Education, the regional epidemiology
units and health services units to intensify the programs due to the
increase in the number of dengue cases.
|


